You should not just ignore this useful information; it is often worth a closer look. Cosmetic, toiletry and perfumery products (cosmetics) are subject to UK laws that ensure that they are safe. These laws also require certain information to be printed or labelled on packaging. This includes an ingredient list, the contents, any warnings that might be necessary on how to use the product safely, and a "period after opening" or a "Best Before Date" to show how long the product may be kept. If it is not obvious by looking at it, the label must state what the product is, for example a body lotion. In addition, the manufacturer will usually give some information about the ingredients used and what to expect from the product.
Most symbols that are used on cosmetics are the same all over Europe. These are easy to understand and have the advantage that words don't have to be translated into a number of different languages and can easily be recognised by consumers wherever they buy their products. A few symbols, such as the bar code or recycling symbol, are commercial in origin but are internationally recognised.
Ingredients
All ingredients used in a cosmetic, toiletry and perfumery product must be listed on the ingredients list. This list is mainly there for people who have been professionally diagnosed with an allergy, so that they can avoid the ingredients to which they are allergic. To avoid these people having to know ingredient names in many different languages, many years ago the industry agreed on a common naming system called the International Nomenclature for Cosmetic Ingredients, or INCI. The same ingredient names are used in every European country and most countries worldwide. Although the names sometimes appear complicated, this is necessary to precisely identify each ingredient and the name is usually simpler than the chemical or botanical name.
 An ingredients list should always appear in the same format and use the same conventions:
An ingredients list should always appear in the same format and use the same conventions:
-
It should be headed by the word INGREDIENTS.
-
Ingredients should be listed in order of weight in the product
-
Ingredient names are from the INCI naming system
-
Perfume mixtures are labelled as "parfum" except for certain specific perfume ingredients which are listed by INCI name
-
Flavours, such as in toothpaste, may be listed as "Aroma"
-
Colours use the Colour Index Number, or CI Number, an international naming system, for example "CI 15580"
For colour cosmetics, such as make-up and lipstick, which come in a range of shades, all of the colours used in the product range are listed together at the end of the list preceded by the "may contain" symbol which is a simple "+/-". Each particular shaded product will use a selection of the colours listed.
Ingredient names to look for on pack
By law, all cosmetic products sold in the UK must display a complete ingredients list. Ingredient labelling helps users to identify products with ingredients to which they know they are allergic. Ingredient names must, by law, comply with UK requirements and use the International Nomenclature of Cosmetic Ingredients, known as INCI. INCI means that in whatever European country a cosmetic product is bought, the ingredient names will be the same. These INCI names have also been adopted by many countries worldwide.
Once opened, use within
 Any cosmetic product that has a lifespan of less than 30 months must show a "Best before the end of" date. This can be shown using the "egg timer" symbol followed by the date, or the words, which can be abbreviated to BBE or Exp, followed by the date.
Any cosmetic product that has a lifespan of less than 30 months must show a "Best before the end of" date. This can be shown using the "egg timer" symbol followed by the date, or the words, which can be abbreviated to BBE or Exp, followed by the date.
For products with a lifespan longer than 30 months, cosmetic products must show a "period after opening" time. That is, the time in months when the product will remain in good condition after the consumer has used the product for the first time. A symbol of an open cream jar is usually used instead of words and the time in months can be inside the symbol or alongside it.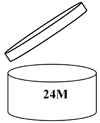
Some products do not require any of these times to be shown because the product will not deteriorate in normal use. Examples are aerosols, which are effectively sealed, perfumes, which have a high alochol content, or single use packs.
Sunscreen labelling
SPF stands for Sun Protection Factor and is an indication of the amount of protection a product provides against UVB light. The SPF number is an industry initiative that has standardised the way a product's UVB protection is indicated throughout Europe and much of the rest of the world.
An SPF indicates the ability of a sun protection product to filter out UVB rays. An SPF of 15 will filter out approximately 93% of UVB rays and an SPF of 30 will filter out around 96%. An SPF of 15 is seen as the recommended minimum by most health experts.
Also alongside the SPF number there will also be an indication of the type of protection that products give you - i.e. low, medium, high or very high.
The SPF numbers you are most likely to see now are shown in the table below.

We should always choose a sunscreen that provides both UVA and UVB protection. The UVA protection that a sunscreen provides will be evident on the label.
 The way that UVA protection is indicated to the consumer has been harmonised. This appears as the letters "UVA" in a circle. This logo will be used throughout Europe, and consumers will know that their product contains at least the recommended minimum level of UVA protection for a sunscreen.
The way that UVA protection is indicated to the consumer has been harmonised. This appears as the letters "UVA" in a circle. This logo will be used throughout Europe, and consumers will know that their product contains at least the recommended minimum level of UVA protection for a sunscreen.
Net Contents
It is a legal requirement to state the net contents of a product on the pack; that is, the quantity of product at the time it is filled into the packaging. For cosmetics, it is shown in grams (g) or millilitres (ml) for solids or liquids respectively. A contents declaration is not required for products whose contents are below 5 g or 5 ml, for single use packs such as sachets or capsules, or for free samples.
 The "e" mark must be shown if the product is filled according to the "average fill system" which is defined in weights & measures legislation.
The "e" mark must be shown if the product is filled according to the "average fill system" which is defined in weights & measures legislation.
So, a typical contents marking for a shampoo would be "200ml e"
Recycling
 The most common symbol seen is the "Green Dot". This is a trade mark that shows membership of a specific recycling and recovery scheme to deal with the packaging waste of the company's products. All companies in Europe and the UK have a legal obligation to recycle and recover packaging waste and the usual way of doing it is to pay a specialist company to do the work on the company's behalf. In the UK, we have a number of competing recovery and recycling schemes and this logo is not used. However, we see the logo on packs in the UK that are also sold in other European countries, such as Ireland for example, where there is only one recovery scheme.
The most common symbol seen is the "Green Dot". This is a trade mark that shows membership of a specific recycling and recovery scheme to deal with the packaging waste of the company's products. All companies in Europe and the UK have a legal obligation to recycle and recover packaging waste and the usual way of doing it is to pay a specialist company to do the work on the company's behalf. In the UK, we have a number of competing recovery and recycling schemes and this logo is not used. However, we see the logo on packs in the UK that are also sold in other European countries, such as Ireland for example, where there is only one recovery scheme.
Further information
 Where there is not enough space to include the ingredients list or warnings and instructions for safe use, the manufacturer will include that information somewhere else in the packaging, on a leaflet for example. The "Hand & Book" symbol shows that information is included elsewhere in the packaging.
Where there is not enough space to include the ingredients list or warnings and instructions for safe use, the manufacturer will include that information somewhere else in the packaging, on a leaflet for example. The "Hand & Book" symbol shows that information is included elsewhere in the packaging.


